More than half of participants taking the highest dose achieved normal A1C levels, a key secondary endpoint in first phase 3 trial of SURPASS program
Participants in this monotherapy study had relatively recently diagnosed diabetes, with a mean duration of 4.7 years
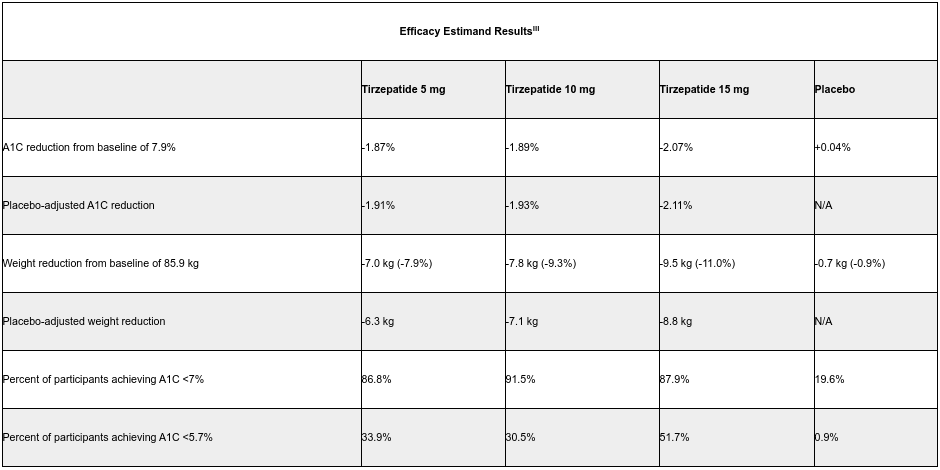
INDIANAPOLIS, Dec. 9, 2020 /PRNewswire/ — Tirzepatide led to superior A1C and body weight reductions from baseline in adults with type 2 diabetes after 40 weeks of treatment in topline results from Eli Lilly and Company’s (NYSE: LLY) SURPASS-1 monotherapy clinical trial evaluating the efficacy and safety of tirzepatide compared to placebo. Using the efficacy estimandi, the highest dose of tirzepatide led to an A1C reduction of 2.07 percent and reduced body weight by 9.5 kg (11.0 percent). More than half (51.7 percent) of participants in this arm achieved an A1C less than 5.7 percent – the level seen in people without diabetes.
The overall safety profile of tirzepatide was similar to the well-established GLP-1 receptor agonist class, with gastrointestinal side effects being the most commonly reported adverse events. Treatment discontinuation rates due to adverse events were less than 7 percent in each tirzepatide treatment arm.
Tirzepatide is a novel investigational once-weekly dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist that integrates the actions of both incretins into a single molecule, representing a new class of medicines being studied for the treatment of type 2 diabetes. The primary and key secondary endpoints of SURPASS-1, the first phase 3 trial of the comprehensive SURPASS program, included superior A1C and mean body weight reductions compared to placebo. Study participants, 54.2 percent of whom were treatment-naïve, had a relatively short mean duration of diabetes of 4.7 years, a baseline A1C of 7.9 percent and a baseline weight of 85.9 kg.
“Tirzepatide delivered impressive A1C and weight reductions for people with type 2 diabetes in this trial, confirming and building upon the phase 2 data that were released in 2018,” said Julio Rosenstock, M.D., Director of the Dallas Diabetes Research Center and Principal Investigator of SURPASS-1. “The study took a bold approach in assessing A1C targets. Not only did nearly 90 percent of all participants taking tirzepatide meet the standard A1C goal of less than 7 percent, more than half taking the highest dose also achieved an A1C less than 5.7 percent, the level seen in people without diabetes – an unprecedented finding and unique endpoint in trials evaluating glucose-lowering agents.”
Treatment differences for two estimands – efficacy and treatment-regimenii – were evaluated for the three tirzepatide doses (5 mg, 10 mg and 15 mg) compared to placebo. Across both estimands, all three tirzepatide doses reached statistical significance in A1C and body weight reductions from baseline and in the percentage of participants who achieved an A1C of less than 7 percent (the American Diabetes Association’s recommended target for people with diabetes) or less than 5.7 percent.
In the treatment-regimen estimand, each of the tirzepatide doses led to statistically significant A1C and body weight reductions:
- A1C reduction: -1.75% (5 mg), -1.71% (10 mg), -1.69% (15 mg), -0.09% (placebo)
- Weight reduction: -6.3 kg (5 mg), -7.0 kg (10 mg), -7.8 kg (15 mg), -1.0 kg (placebo)
- Percentage of participants achieving A1C <7%: 81.8% (5 mg), 84.5% (10 mg), 78.3% (15 mg), 23.0% (placebo)
- Percentage of participants achieving A1C <5.7%: 30.9% (5 mg), 26.8% (10 mg), 38.4% (15 mg), 1.4% (placebo)
No events of severe hypoglycemia or hypoglycemia less than 54 mg/dL were observed in the tirzepatide treatment arms.
The most commonly reported adverse events were gastrointestinal-related and mild to moderate in severity, usually occurring during the dose escalation period. For study participants treated with tirzepatide (5 mg, 10 mg and 15 mg, respectively), nausea (11.6 percent, 13.2 percent, 18.2 percent), diarrhea (11.6 percent, 14.0 percent, 11.6 percent), vomiting (3.3 percent, 2.5 percent, 5.8 percent) and constipation (5.8 percent, 5.0 percent, 6.6 percent) were more frequently experienced compared to placebo (6.1 percent [nausea], 7.8 percent [diarrhea], 1.7 percent [vomiting], 0.9 percent [constipation]). The overall treatment discontinuation rates were 9.1 percent (5 mg), 9.9 percent (10 mg), 21.5 percent (15 mg) and 14.8 percent (placebo). The majority of the discontinuations in the 15 mg and placebo arms were due to reasons other than adverse events (such as concerns due to the coronavirus pandemic and family or work reasons).
“As a leader in diabetes care, we have a nearly 100-year heritage of innovating to advance care for people living with diabetes. Tirzepatide is the first dual GIP/GLP-1 receptor agonist to complete a phase 3 trial,” said Mike Mason, president, Lilly Diabetes. “We are impressed by these initial results showing how tirzepatide performed in people with a relatively short duration of diabetes, and we look forward to seeing more results in people who are later in the course of diabetes in future studies from our robust SURPASS clinical trial program.”
The complete SURPASS-1 study data have not yet been evaluated but will be presented at the American Diabetes Association’s® 81st Scientific Sessions® and published in a peer-reviewed publication in 2021.
About tirzepatide
Tirzepatide is a once-weekly dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist that integrates the actions of both incretins into a single novel molecule. GIP is a hormone that may complement the effects of GLP-1 receptor agonists. In preclinical models, GIP has been shown to decrease food intake and increase energy expenditure therefore resulting in weight reductions, and when combined with a GLP-1 receptor agonist, may result in greater effects on glucose and body weight. Tirzepatide is in phase 3 development for blood glucose management in adults with type 2 diabetes and for chronic weight management. It is also being studied as a potential treatment for non-alcoholic steatohepatitis (NASH).
About SURPASS-1 and the SURPASS clinical trial program
SURPASS-1 (NCT03954834) is a 40-week, multi-center, randomized, double-blind, parallel, placebo-controlled trial comparing the efficacy and safety of tirzepatide 5 mg, 10 mg and 15 mg as monotherapy to placebo in adults with type 2 diabetes inadequately controlled with diet and exercise alone. The trial randomized 478 study participants across the U.S., Mexico, India and Japan in 1:1:1:1 ratio to receive either tirzepatide 5 mg, 10 mg or 15 mg or placebo. The objective of the study was to demonstrate that tirzepatide (5 mg, 10 mg or 15 mg) is superior in A1C reduction from baseline after 40 weeks in people with type 2 diabetes naïve to injectable therapy who haven’t used any oral antidiabetic medicines within three months compared to placebo. Study participants had a mean A1C between 7 percent and 9.5 percent and a BMI greater than or equal to 23 kg/m2. All participants in the tirzepatide treatment arms started the study at a dose of tirzepatide 2.5 mg once-weekly and then increased the dose in a step-wise approach at four-week intervals to their final randomized maintenance dose of 5 mg (via a 2.5 mg step), 10 mg (via steps at 2.5 mg, 5 mg and 7.5 mg) or 15 mg (via steps at 2.5 mg, 5 mg, 7.5 mg, 10 mg and 12.5 mg).
The SURPASS phase 3 global clinical development program for tirzepatide has enrolled more than 10,000 people with type 2 diabetes across eight clinical trials, five of which are global registration studies. The program began in late 2018 with results anticipated in late 2020 and 2021.
About Diabetes
Approximately 34 million Americans1 (just over 1 in 10) and an estimated 463 million adults worldwide2 have diabetes. Type 2 diabetes is the most common type internationally, accounting for an estimated 90 to 95 percent of all diabetes cases in the United States alone1. Diabetes is a chronic disease that occurs when the body does not properly produce or use the hormone insulin.
About Lilly Diabetes
Lilly has been a global leader in diabetes care since 1923, when we introduced the world’s first commercial insulin. Today we are building upon this heritage by working to meet the diverse needs of people with diabetes and those who care for them. Through research, collaboration and quality manufacturing we strive to make life better for people affected by diabetes and related conditions. We work to deliver breakthrough outcomes through innovative solutions—from medicines and technologies to support programs and more. For the latest updates, visit http://www.lillydiabetes.com/ or follow us on Twitter: @LillyDiabetes and Facebook: LillyDiabetesUS.
About Eli Lilly and Company
Lilly is a global healthcare leader that unites caring with discovery to make life better for people around the world. We were founded more than a century ago by a man committed to creating high-quality medicines that meet real needs, and today we remain true to that mission in all our work. Across the globe, Lilly employees work to discover and bring life-changing medicines to those who need them, improve the understanding and management of disease, and give back to communities through philanthropy and volunteerism. To learn more about Lilly, please visit us at lilly.com and lilly.com/newsroom. P-LLY
This press release contains forward-looking statements (as that term is defined in the Private Securities Litigation Reform Act of 1995) about tirzepatide as a potential treatment for people with type 2 diabetes and the timeline for future readouts, presentations and other milestones relating to tirzepatide and its clinical trials, and reflects Lilly’s current belief and expectations. However, as with any pharmaceutical product, there are substantial risks and uncertainties in the process of research development and commercialization. Among other things, there can be no guarantee that the studies will be completed as planned, that future study results will be consistent with the results to date or that tirzepatide will receive regulatory approvals. For further discussion of these and other risks and uncertainties, see Lilly’s most recent Form 10-K and Form 10-Q filings with the United States Securities and Exchange Commission. Except as required by law, Lilly undertakes no duty to update forward-looking statements to reflect events after the date of this release.
1 Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept. of Health and Human Services; 2020.
2 International Diabetes Federation. IDF Diabetes Atlas, 9th edn. Brussels, Belgium: International Diabetes Federation, 2019. Available at: http://diabetesatlas.org.
i Efficacy estimand represents efficacy prior to discontinuation of study drug or initiating rescue therapy for persistent severe hyperglycemia.
ii Treatment-regimen estimand represents the efficacy irrespective of adherence to the investigational medicine or introduction of rescue therapy for persistent severe hyperglycemia.
iii All three tirzepatide doses led to statistically significant A1C and body weight reductions from baseline and also reached statistical significance in the percentage of participants who achieved an A1C of less than 7 percent or less than 5.7 percent.
View original content to download multimedia:http://www.prnewswire.com/news-releases/lillys-tirzepatide-significantly-reduced-a1c-and-body-weight-in-people-with-type-2-diabetes-301188988.html
SOURCE Eli Lilly and Company


Recent Comments