This post was updated on March 27, 2024 to include a clarification of the drugs included in the analysis and additional information about rebates.
GLP-1 drugs such as Ozempic, Wegovy, and Mounjaro were initially developed to treat type 2 diabetes, but their effectiveness as anti-obesity medications has generated tremendous excitement and high demand among people who have struggled to lose weight by other means. These drugs are also being tested to treat other conditions, and the FDA has just approved a new use for Wegovy to reduce the risk of adverse cardiovascular events. But the annual cost of these drugs in the US – upwards of $11,000 at recent list prices, though net prices may be lower with rebates negotiated by pharmacy benefit managers – has raised concerns about the fiscal impact of broad coverage of GLP-1 drugs on Medicare, other health insurers, and patients.
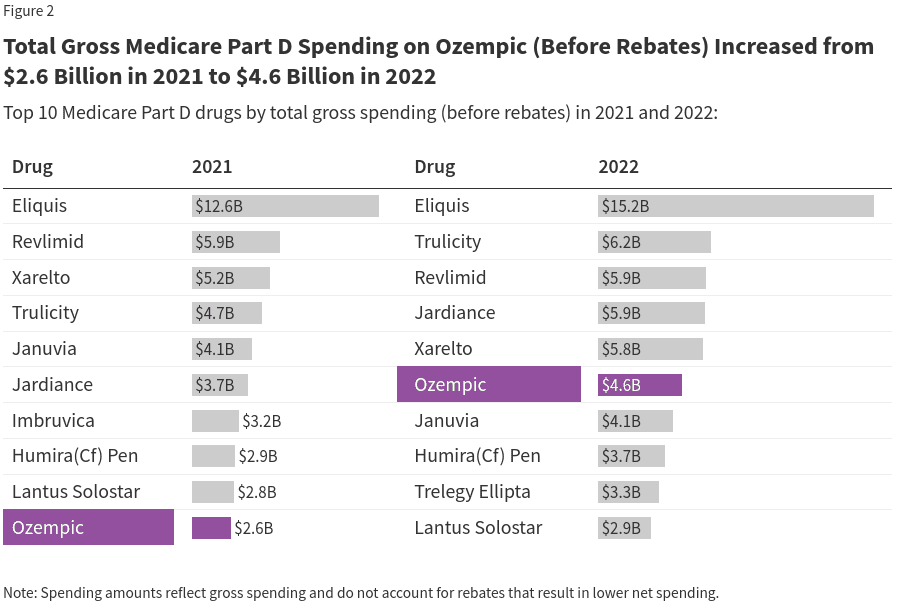
Medicare is prohibited under current law from covering drugs used for weight loss, but Medicare Part D plans can cover GLP-1s for their other medically-accepted indications, including to treat diabetes, and now to cut cardiovascular risk based on a recent memo from the Centers for Medicare & Medicaid Services (CMS). While the potential cost of authorizing Medicare coverage of anti-obesity drugs has presented a barrier to enacting legislation to lift the prohibition, covering these drugs under Medicare for authorized uses has already catapulted these drugs to rank among the top-selling drugs covered by Part D, Medicare’s outpatient drug benefit program.
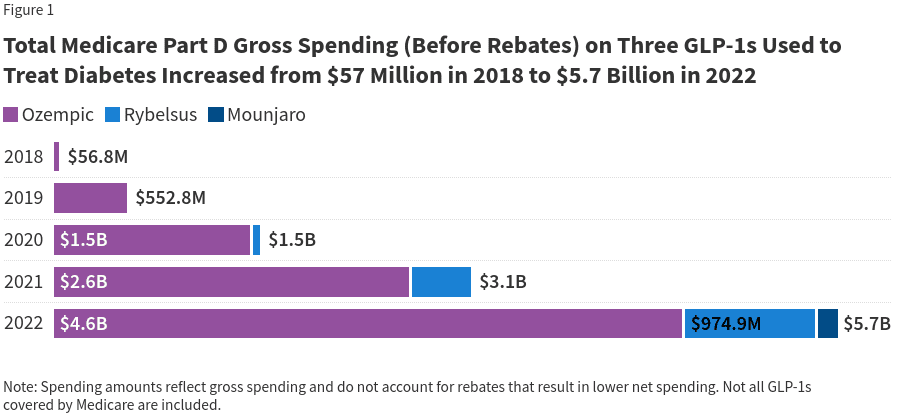
KFF’s analysis of newly released Medicare Part D spending data from CMS shows that total gross Medicare spending on the three newest versions of these diabetes medications that have also been recently approved for weight loss – Ozempic, Rybelsus, and Mounjaro – has skyrocketed in recent years, rising from $57 million in 2018 to $5.7 billion in 2022 (Figure 1). (Gross spending does not account for rebates that result in lower net spending.) Ozempic (semaglutide injection) was approved in December 2017; Rybelsus (semaglutide tablets) was approved in September 2019; and Mounjaro (tirzepatide) was approved in May 2022. As weight loss drugs, semaglutide was approved as Wegovy in 2021 and tirzepatide was approved as Zepbound in 2023. (This analysis does not include all GLP-1s covered by Medicare, only those products with more recent FDA approvals that are also approved as weight loss medications.)
Juliette Cubanski and Tricia Neuman
Published: Mar 22, 2024
Original Article Here.


Recent Comments